Lux Skin & Lasers
ULTHERAPY VS SOFWAVE
THE LIFT YOU CAN SEE™
Ultherapy’s real-time visualization and multi-depth targeting ensures precise energy delivery for safe, accurate, and reproducible results, suitable for all skin types.1,2

SAFETY FIRST:
PROTECTING PATIENTS
Extensive Safety Data: Ultherapy’s Evidence Edge Over Sofwave.
ULTHERAPY IS SAFE FOR ALL SKIN TYPES






Not actual patients.
NO SIGNIFICANT DIFFERENCE IN PAIN LEVELS3
Ultherapy patients report pain levels of 5.6/10 for forehead, 3.9/10 for temple, and 6.09/10 for submental areas
SAFE ENERGY DELIVERY1,3
Ultherapy’s precise energy delivery allows practitioners to target 3 depths of collagen-rich layers without the need for additional
cooling
REAL-TIME PRECISION1,2
Ultherapy’s real-time imaging helps practitioners avoid structures like bones and blood vessels while targeting collagen-rich tissue
VS SOFWAVE3
Sofwave patients report pain levels of 6.4/10 for forehead and 5.3/10 for neck/submental areas
VS SOFWAVE3
Sofwave necessitates an integrated cooling tip for epidermal protection
VS SOFWAVE3
Without visualization, practitioners risk complications from improper energy delivery, potentially causing adverse events and increased pain
TREATMENT DEPTH:
1 TREATMENT, 3 DEPTHS
Ultherapy® offers a triple-depth advantage compared to Sofwave’s single-depth treatment.1,3,4
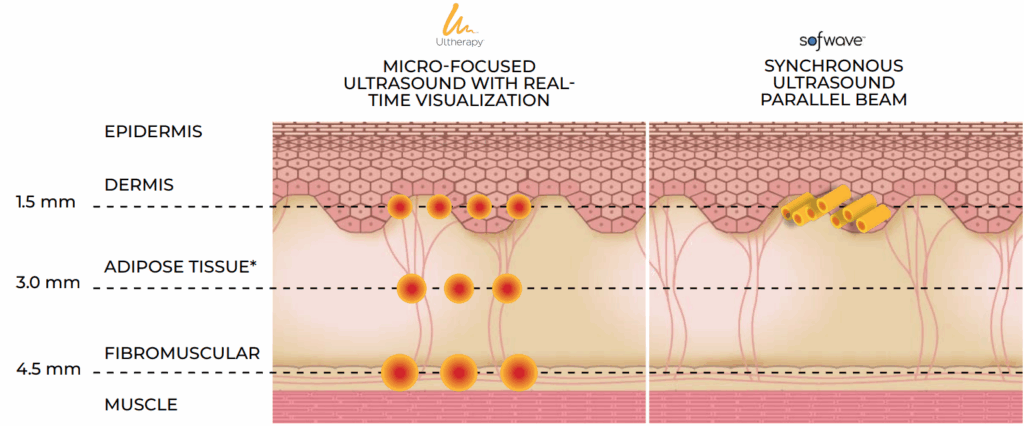
*For illustrative purposes only, as individual skin layers vary in depth. Do not deliver energy solely to adipose tissue.
DEEPER TREATMENT1,4,5
Ultherapy offers targeting at depths of 1.5, 3.0, and 4.5 mm, reaching both the deep dermis and collagen-rich fibrous layers
MULTI-DEPTH PRECISION5,6
Ultherapy ensures accurate treatment at the same tissue planes as a modern facelift, suitable for all skin types
CONSISTENT, REPRODUCIBLE RESULTS1,2
Ultherapy consistently delivers precise energy without affecting surrounding tissue
VS SOFWAVE3-5
Sofwave’s SUPERB™ technology only targets 1.5 mm, unable to reach deeper, collagen-rich fibrous layers
VS SOFWAVE7
Sofwave’s SUPERB technology may induce thermal injury at depths ranging from 0.5 to 2 mm
VS SOFWAVE3
Sofwave’s limited depth targeting
and lack of real-time visualization
may result in less precise treatment, potentially affecting
surrounding tissue
TRUST ULTHERAPY FOR A MULTI-DEPTH LIFT
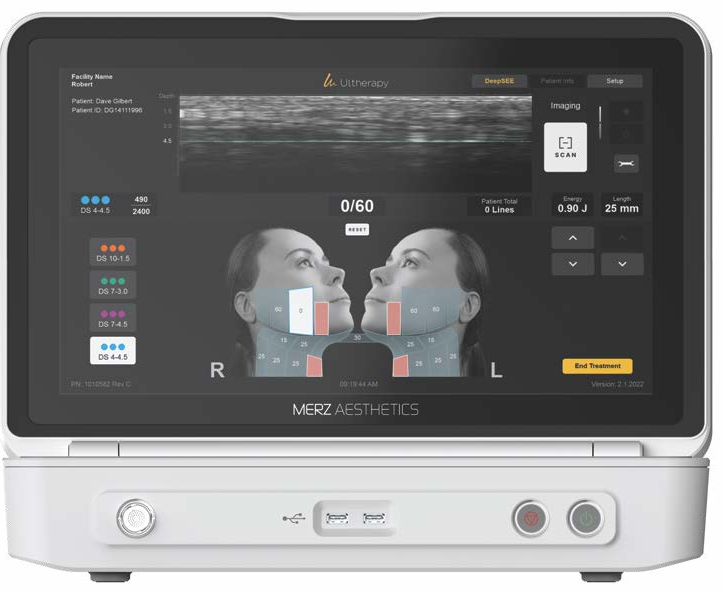
REAL-TIME IMAGING:
VISUALIZE TO OPTIMIZE
Leverage real-time visualization to promote more personalized and effective treatments.
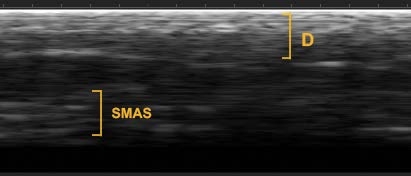
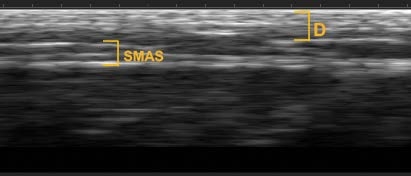
TISSUE LAYER DEPTH VARIES ACROSS PATIENTS AND ANATOMICAL REGIONS
Treatment Example: Cheek Region
ULTHERAPY IS SAFE FOR ALL SKIN TYPES

- 52 years old
- Caucasian
- Male
- 6’ 1”
- 207 pounds

- 43 years old
- Pacific Islander
- Female
- 5’ 9”
- 141 pounds
Not actual patients.
CUSTOMIZED TREATMENTS1,2
Practitioners control where energy is deposited with Ultherapy and can adapt to patient anatomy to customize treatment and deliver precise results
VISUALIZATION FOR TARGETED TREATMENT DEPTH1,2,8,9
Ultherapy enables practitioners to visualize skin layers and deliver energy precisely where needed, tailoring treatment to each patient’s anatomy
VS SOFWAVE3
Sofwave cannot assess patient anatomical variances
under the skin, limiting the customization of treatments
VS SOFWAVE3
Without visualization and targeting only 1 treatment depth, providers cannot customize treatment
14+ years
of market presence3
50+ published clinical studies
120+ publications3
2.6+ million treatments
performed in 80+ countries worldwide3
HIGH PATIENT SATISFACTION110
Ultherapy boasts robust clinical evidence and a 95% patient satisfaction rate at 1 year
1 SESSION, 0 DOWNTIME3,10
Ultherapy provides a long-lasting lift after a single 30- to 90-minute treatment, lasting up to a year or more
FEWER TREATMENTS OVER TIME99,10
Ultherapy’s efficacy extends far beyond the initial session, with multiple studies demonstrating improvements in skin laxity lasting up to a year or more
VS SOFWAVE3
Sofwave lacks extensive long-term data and comparable patient satisfaction rates
VS SOFWAVE3,11
Sofwave may require multiple treatments for results lasting up to 6 months
VS SOFWAVE3,11
As of May 2024, only 1 small study of Sofwave (N=15) demonstrates 6-month improvement, with no additional long-term data
| Feature | Ultherapy | Sofwave |
|---|---|---|
| Multi-depth treatment down to the SMAS1,3,5 | ✔️ | |
| Real-time visualization1,3 | ✔️ | |
| Precision & customization1-3,8 | ✔️ | |
| Results lasting up to a year or more3,10 | ✔️ | |
| Single-session treatment3,10 | ✔️ | |
| No downtime3,14 | ✔️ | ✖️ |
| Effective for all Fitzpatrick skin types4,14 | ✔️ | ✖️ |
ADVANCED
Ultherapy PRIME’s ADVANCED platform features a faster and more powerful processing performance, elevated ergonomics, and sophisticated design
VIVID
Ultherapy PRIME platform is the only FDA-cleared, non-invasive lifting device with VIVID real-time imaging that allows practitioners to assess their patients’ unique needs to promote safer and more effective results.1,2,5,6,8,9,13
PROVEN
Ultherapy PRIME platform builds on the legacy of PROVEN, safe, and effective results, with more than 120 publications, 56 clinical studies, and more than 2.6 million treatments performed in more than 80 countries.3,9,10
The ULTHERA® System is indicated to lift the skin on the neck, on the eyebrow, and under the chin as well as to improve lines and wrinkles on the décolleté. Reported adverse events from post marketing surveillance are available in the Instructions for Use (IFU). Please see the IFU for product and safety information, and possible side effects at Ultherapy.com/IFU.
6 MONTHS VS 1 YEAR: ULTHERAPY PRIME’S LIFT LASTS LONGER
REFERENCES
1. Ultherapy [Instructions for Use]. Mesa, AZ: Ulthera, Inc; 2022.
2. Pavicic T, et al. J Cosmet Dermatol. 2022;21(2):636-647.
3. Data on file. Merz North America, Inc. US-ULT-2300029.
4. Fabi SG. Clin Cosmet Investig Dermatol. 2015;8:47-52.
5. Suh DH, et al. J Cosmet Laser Ther. 2015;17(5):230-236.
6. Whitney ZB, et al. In: StatPearls [Internet]. Updated January 30, 2024. Accessed July 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK519014/
7. Data on file. Sofwave Inc. PB00041-2.
8. Fabi SG, et al. J Drugs Dermatol. 2019;18(5):426-432.
9. Park JY, et al. J Clin Aesthet Dermatol. 2021;14(5):E70-E79.
10. Werschler WP, Werschler PS. J Clin Aesthet Dermatol. 2016;9(2):27-33.
11. Gold MH, Biron J. J Cosmet Dermatol. 2024;23(1):117-123.
12. Data on file. Merz North America, Inc. GL-ULT-2300002.
13. Fabi SG, et al. J Am Acad Dermatol. 2013;69(6):965-971.
14. Data on file. Sofwave Inc. PB00030-4.
TRUST ULTHERAPY: THE GOLD STANDARD IN NON-INVASIVE LIFTING AND TIGHTENING
© 2024 Merz North America, Inc. All rights reserved. ULTHERA, ULTHERAPY, ULTHERAPY PRIME, the ULTHERAPY Logo, and the SQUIGGLE Logo are registered trademarks, and THE LIFT YOU CAN SEE is a pending trademark, of Ulthera, Inc. in the U.S. MERZ AESTHETICS is a registered trademark of Merz Pharma GmbH & Co. KGaA in the U.S. All other marks are the property of their respective owners. US-ULT-2400117.


